As I might have mentioned already, my favorite protists are the shaggy, shapely, fast-moving ciliates. They have a lot to offer the idle protist-ogler. As a group, they include some of the largest and most ridiculous-looking microbes in the pond. Many are easy to identify without expensive equipment or special techniques. Some, like the noodle-necked Lacrymaria olor, can be recognized at a glance in the light microscope, even at low magnification. Others, like the stately Stentors, may need closer inspection for a species-level classification, but can still be identified by prominent features such as the colour of the cell, or the shape and distribution of various organelles.
Unfortunately, not all ciliates are so easy to tell apart. Some are like the “little brown birds” that plague neophyte birders, and can only be distinguished from one another by very close observation under exacting conditions. And many, I’m sorry to say, are pretty much impossible to identify, even to genus level, without the help of special stains that expose distinctive patterns in the cilia on the surface of the cell body.
The classic technique for exposing these structures is to fix the cells in some noxious and foul-smelling substance and then soak them in solutions containing various compounds of silver. Certain parts of the organism–most conveniently, for our purposes, the ciliary rows and the nuclei–are “argentophilic,” which is to say they stain darkly when exposed to silver. The ability to selectively stain these organelles revolutionized ciliate taxonomy in the second half of the 20th century, and it is still the most important technique available to modern ciliatology.
Despite my particular interest in ciliates, I’d never tried it until just a few days ago.
I’ve been slow to get around to this, mainly because it’s hard to do. Even the easiest methods of silver staining call for a cupboard full of powders and solvents, none of which is available at Shopper’s Drug Mart, and some of which must be handled and stored very thoughtfully. To procure the ingredients I had to find suppliers willing to do business with an individual buyer, and in some cases I had to pay special transport fees.
Then, of course, I had to assemble the equipment required to use this stuff safely: graduated cylinders, flasks, funnels, fixing jars, an accurate scale, syringes, latex gloves, etc.
And finally, I had to acquire a bunch of new skills. I haven’t stood at a lab bench since the ninth grade (40 years ago, if you can believe it), so I had to learn how to do simple tasks, like weighing, pouring and mixing. Fortunately, before undertaking any of this, I had the foresight to culture a full-sized biochemist, which was quite expensive and took about 23 years. He is currently living in my basement, and was very helpful at several points.
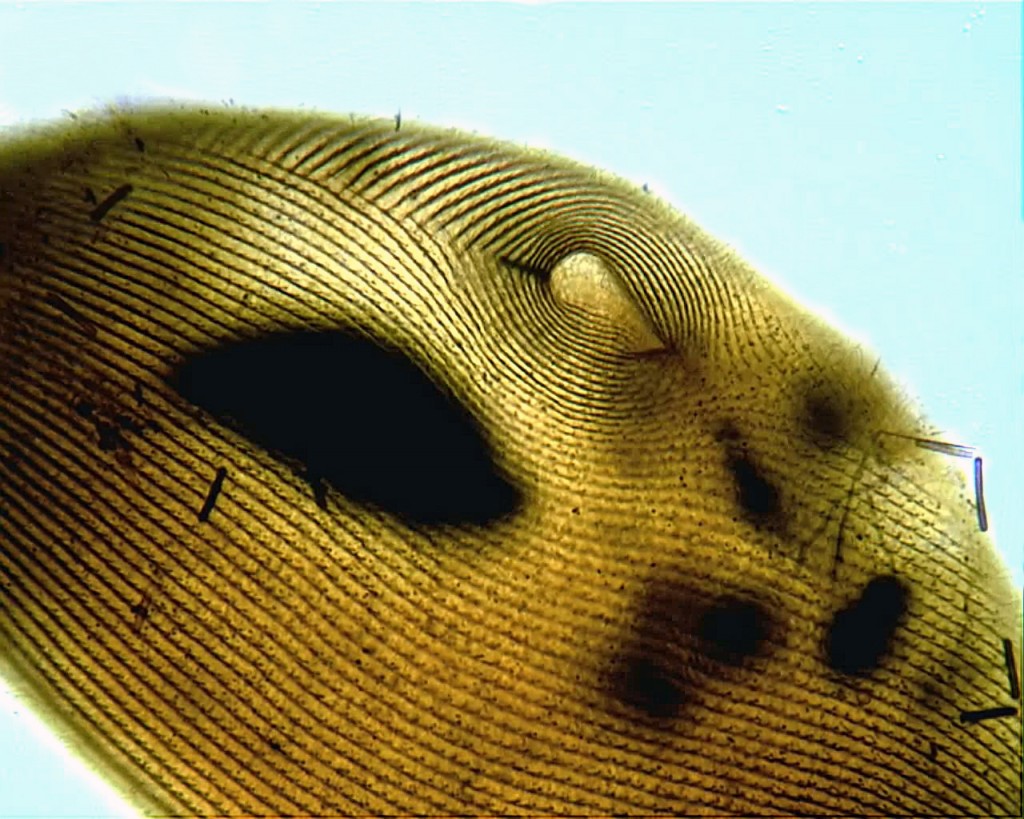
Here, then, is my first attempt at staining a plain old Paramecium by one of the silver carbonate methods:
Yes, there’s a lot of room for improvement, but, frankly, I’m delighted that it worked at all.
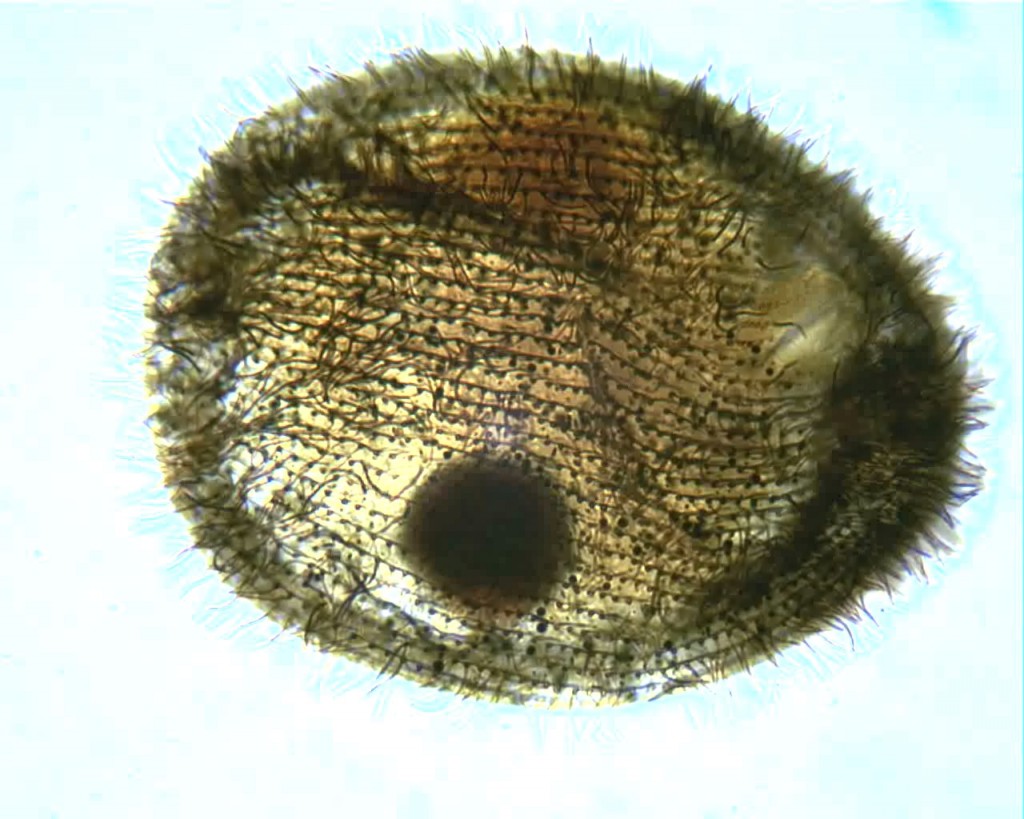
Here’s one more from the same slide, a specimen of a common hymenostome ciliate with the curious name of Glaucoma:
The impregnation could be more uniform, the focus could be sharper and the hot spot from the microscope lamp is downright annoying. However, I can count the kineties and easily see the shape of the macronucleus. That’s a step forward, for me.
The protocol I followed is the one developed by Augustin, Foissner and Adam in 1984 (described in Foissner’s updated guide to basic methods for ciliate taxonomy). I’m told that the original Fernandez-Galiano method gives more consistent results, so I’ll try that next.



Congratulations!
i think that experience and some luck are key when silver staining rather than the exact formula used. Also, I feel that the physiological state of the cell at the time of staining is important. If you have the patience and sufficient material, it might be worth trying slight variations of technique (eg timings) with different portions of the sample. i think your preps look good and are probably sufficient for ID ….but perhaps you seek perfection!
BTW congratulations as well for brewing a biochemist… shows great forethought and patience.
regards,
Brian
Thanks for the encouragement! Yes, perfection would be good…but I guess I’d settle for consistency. Some cells impregnated really well, while others of the same species refused to take up the stain (I’m guessing that’s a fixation issue). Ultimately, I’d like to resolve fine details, like the dorsal brosse on some ciliates, as well as the coarser features.This experiment used rugged hymenostomes and peniculids…I’m hoping I can work with certain dainty litostomateans without exploding them. I’ve been told this is a bit of a black art, so I’ll keep playing around with different variables (like number of drops of Fernandez-Galiano fluid and the amount of dark-staining flocculent crud in the sample) and try to be organized enough to record my timings.
Nice work, I am looking to do the same thing and it is encouraging that this worked for someone else without much lab experience.
I have a few questions:
1. Did you have any special safety procedures? I read that it is recommended to handle formalin in a fume hood, was this necessary? I think it would be fine without one in a well ventilated area if only a small amount of formalin is used.
2. If you don’t mind sharing, how much did all the chemicals cost? Glassware and other equipment excluded.
3. Have you learned any tricks since writing this article? This will be my first experience with staining 🙂
Thank you very much for writing this.
Regards,
Luke
Hi Luke,
Sorry for the slow answer. My blog software hasn’t been notifying me of messages, for some reason.
It is an extremely good idea to use a fume hood, and I plan to build one. Silver carbonate staining does require the use of some unwholesome substances, like formalin and pyridine. Fortunately, the amounts are very small. When using these reagents, I set up my equipment in the window well of a ventilated room. I place a fan next to my mixing area, to pull any fumes straight out the window, and after working I run a ceiling fan for twenty minutes, with the door closed, after I’ve finished mixing. Pyridine has a strong smell, so it’s easy to tell when it is in the air. I work over absorbent paper, but am also careful not to spill so much as a drop. Needless to say, I also wear gloves, goggles and labcoat, and am meticulous about storing materials and equipment.
I’ll send you an email about the costs of reagents. I need to look up the amounts.
I hope to do another run this weekend, so I’ll let you know if I learn any new tricks! I’ve been discussing the process with a fellow ciliate enthusiast, and try some of his suggestions. If all goes well, I’ll post a followup.
cheers,
Bruce
Nice results! I am thinking of doing the same thing. From what I’m reading I need proteose peptone, did you use any? In the picture I see peptone, is there a difference?
Thanks,
Luke
Thanks, Luke!
I don’t know if there is a significant difference between the generic peptone I’m using and the brand of proteose peptone recommended in the protocols I’ve been following. The recommended brands were available only in large quantities, at horribly high cost, so I hope this stuff will do.
best,
Bruce
Hi
This “blocking” reagent is important but peptone should give similar results to proteose peptone.
Brand is not important. Varying quantities is useful for trials and errors.
The most important is the quality of silver nitrate and mostly pyridine. Don’t forget to work under a fume hood or wear a full coverage mask as Formaldehyde and Pyridine are extremely toxic ! (carcinogenic and very dangerous for the eyes).
Thanks for the clarification about peptone, Phil! I agree about the importance of using a fume hood. Anyone who undertakes this kind of work should do so in a well-equipped lab, if possible, with the guidance of an experienced technician. My oldest boy has been my advisor on laboratory procedures, and his help was indispensable. My makeshift ventilation system works very well, but is only a temporary solution, until I build an efficient fume hood.
I was expecting some photos from your first attempts. Congratulations ! It looks very promising.
My advice for easy staining: try Holophrya, Colpidium, Colpoda or even Urocentrum. They stain very easily
Paramecium, Frontonia as well but they tend to darken very fast.
Play around with component ratio is a good Idea. I am now using microvolumes and microtubes.
Phil
Hi Phil,
I didn’t see this message until today. I think I forgot to tick some email notification boxes in my blog software.
“Darkening fast” hasn’t been a problem for me. Getting sufficient impregnation has been more difficult. I’ll follow the excellent suggestions you provided on the photomacrography forum, and see if my results are more uniform.
Your superb Holophyra images have inspired me to do another run, provided I can gather enough ciliates from my stale jars (all my ponds are under ice!).
cheers,
Bruce