As I was saying, we vertebrates tend to have a high opinion of the big clump of neurons that sits at one end of our spinal cord. And so we should: in creatures like us, that’s where the magic happens. The bigger the clump, the more cognitively versatile the organism; remove it, and the whole organism comes to a stop (though it may run around for a while, first).
But, as we know, even unicellular beings are capable of sensation and coordinated movement, and can show complex, even purposeful, behaviours. So, it was probably inevitable that some well-meaning scientist would try to equip them with a central nervous system of their own.
Stylonychia mytilus crawling on a filament of algae. Image by Actinophrys, on Flickr. Click for Link.
In 1880, a German researcher named Th. W. Engelmann undertook a close inspection of the tissues of the ciliate Stylonychia mytilus. Like other members of the ciliate group loosely known as “hypotrichs,” Stylonychia has, on its “belly,” a crop of thick, mobile cirri: bundles of fused cilia that it uses like little legs, as it scrambles around on solid objects in its sunken world. These pseudo-legs work very well, and a crawling hypotrich can look a lot like a beetle or cockroach.
Unlike the limbs of insects, the cirri of hypotrichs lack conspicuous attachments, such as muscles, tendons and nerves. However, looking very closely at stained samples, Engelmann was able to detect, at the bases of Stylonychia’s cirri, groups of fibres which he called “Wimperwurzeln,” or “ciliary rootlets.” These fibres, he speculated, might just function as nerves do in “higher” organisms, serving as the conductive tracks along which the stimuli that move the cilia might move!
It was an innovative idea; however, it did not spark much excitement. Despite his impressively clear record of these small structures, the suggestion that he had discovered animal-like organs in unicellular creatures might have come at the wrong time. By 1880, the view of protozoa as miniature animals, bearing a complete set of reproductive and alimentary organs, had been discredited a long time before. In the late 19th century, protozoa were conventionally seen as primitive organisms, little more than bags of the all-purpose protoplasm that constituted, in Thomas Huxley’s memorable phrase, the “physical basis of life.”
But times change, and the idea that free-living cells might be composed of differentiated tissues would came back into style (in fact, in the early 20th century some protozoologists completely rejected the cellular nature of their organisms).
In 1914, Robert G. Sharp–working in California under the guidance of the eminent protozoologist Charles Atwood Kofoid–published a paper in which he boldly claimed to have found a ciliate that not only had nerve-fibres, but a full-blown centrally located “neuromotor apparatus.” He discovered this structure in an organism called Diplodinium ecaudatum (now known as Epidinium ecaudatum), a symbiont in the stomachs of cattle. What Sharp found had never been recorded anywhere: a small mass of fibres close to the cell’s motile organelles, and serving as “the common center of motor influences.” He had discovered the organ that controlled and coordinated the moving parts of the ciliate, in effect, the cytoplasmic “brain” of the cell. He called this the “motorium.”
Sharp’s paper kicked up a little whirlwind of scientific activity, particularly among his colleagues at Kofoid’s laboratory. The following year, Kofoid himself described a “neuromotor apparatus” in the intestinal parasite, Giardia. Shortly after, Harry B. Yocom, another worker in Kofoid’s group, published the discovery of a full-blown neuromotorium in the hypotrich Euplotes patella (a “walking ciliate” like Stylonychia).
But why ascribe a neural function to the structures he saw in Euplotes? Yocom goes into some detail about that. In the first place, these fibres turn a vivid red in the fuchsin from Mallory’s stain, just as nerve cells do in metazoa; in the second place, he explains, these fibres are found in association with the moving parts of the ciliate: the oral membranelles and the locomotory cirri.
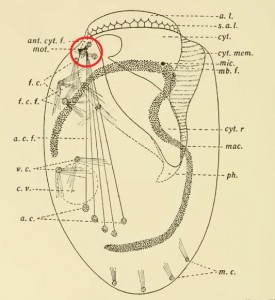
Yocom’s illustration of the neuromotor apparatus in Euplotes patella, with the “motorium” circled in red.
Finally, the behaviour of Euplotes patella appeared to support this interpretation. It is, perhaps, a classic example of hypothesis leading observation by the nose. To Yocom, it seemed evident that E. patella was using its “anterior lip” (the bulge at the top of the cell, which showed a red-staining lattice of fibres) as a sensory organ. What’s more, the transverse cirri, which were linked to the “motorium,” moved in a coordinated way, while the unattached cirri moved randomly. As Yocom puts it: “[T]he whirling irregular movements of the frontal ventral and marginal cirri are in no way coordinated with the regular rhythmical movements of the membranelles or with the backward kick of the cirri.”
Or, so it looked to him.
Up to that point, the function of the “neuromotorium” as a “coordinating center” for the cell’s movements was simply a hypothesis, unsupported by experiment. But confirmation was not long in coming. In 1919, C. V. Taylor–yet another worker from the Berkeley circle–conducted an experiment on Euplotes patella, the ciliate Yocom had studied in such detail the year before. Using quartz microscalpels, he severed the fibres running between the motorium and the anal cirri (the prominent group of five heavy cirri in the posterior half of the cell, now usually known as “transverse cirri”). The experiment resoundingly confirmed the hypothesis; detached from the motorium, the cirri did not seem to work properly at all: “Severing the fibers to the anal cirri affects both creeping and swimming…Destroying the motorium or cutting its attached fibers interrupts coordination in the movements of the adoral membranelles and anal cirri.” (Taylor, 1919)
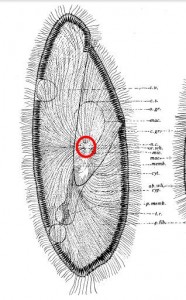
Paramecium from Rees (1920), showing a tracery of fine “neurofibrils” (skeletal microtubules, presumably) converging on the “nerve center” of the cell, circled here in red.
From then on, the neuromotor apparatus turned up in one ciliate after another. It was found in Paramecium (1920), Balantidium (1922), Tintinnopsis (1926), Dileptus (1927), Chlamydodon (1928), Uroleptus (1930), and Oxytricha (1935). Along the way, doubt steadily faded, and by 1927 it was possible to affirm plainly that “a complicated neuromotor system has been conclusively demonstrated to exist in certain ciliated infusoria.” (Visscher, 1927) It had become, in North America, at least, a solid fact.
But even solid facts can turn fluid again, and then evaporate like the canals of Mars. It can happen slowly, or all at once. Sometimes a hypothesis dies a quick death, when an experiment disproves it; sometimes it is swept off in the riptide of a better theory; and sometimes it just get nibbled away, while the world slowly changes around it, until one day nobody can remember why it had ever seemed true.
The neuromotor concept had a long run, outlasting Kofoid himself by a decade and a half. Even the advent of electron microscopy did not kill it off, right away. As late as 1957, EM seemed to reveal in Euplotes “a mass of intertwining rootlet filaments” corresponding to the neuromotorium, as well as a whole system of intracellular fibres which were mainly dedicated, as the authors argued, to “the coordination of the ciliary beat.” (Roth, 1957) But when R. Gliddon again placed Euplotes under the electron microscope, in 1966, he could not find anything you could call a motorium. It had disappeared.
In that same year, two researchers in Japan finally got around to replicating Taylor’s classic experiment on E. patella–the experiment that, arguably, had kicked off the neuromotorium gold rush. (Okajima and Kinosita, 1966) But now, the results were very different. The investigators severed the “neurofibrils,” just as Taylor had done, and captured the ciliary movements on film; however, this time the cirri just kept on moving in their usual coordinated way, as if nothing had happened. It is not known why Taylor’s Euplotes had behaved differently. Possibly, he mangled the poor things while dissecting their “neurofibrils” and made his celebrated observations on organisms in their death throes.
In 1970, Dorothy Pitelka was ready to call the game, announcing the general “abandonment of the old concept of a fibrillar neuromotor system in ciliates.” A casual search in Google Books turns up one last, brief reference to the “neural fibrils” of Paramecium, in a high school biology textbook published in 1983. After that, nothing.
So what were the “neurofibrils,” in the end? They were not a product of poor equipment or fanciful observation, but rather the opposite. Remarkably, it seems that microscopists of the day were already detecting parts of the cytoskeleton–the scaffolding of microtubules and filaments that give the cell its shape–as well as the pellicular “latticework,” the so-called ciliate “silverline system” which would later be fully exposed in silver nitrate and protargol preparations. Interpretation lagged behind observation for a generation or two. Seeing had outstripped understanding; then new information came, and the old certainties just sank back into the pondwater.