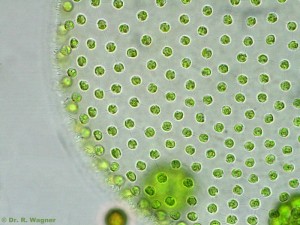
Early last year, the mayor of Salzburg proudly announced the creation of a new conservation zone around this “globally unique ‘natural monument'”:
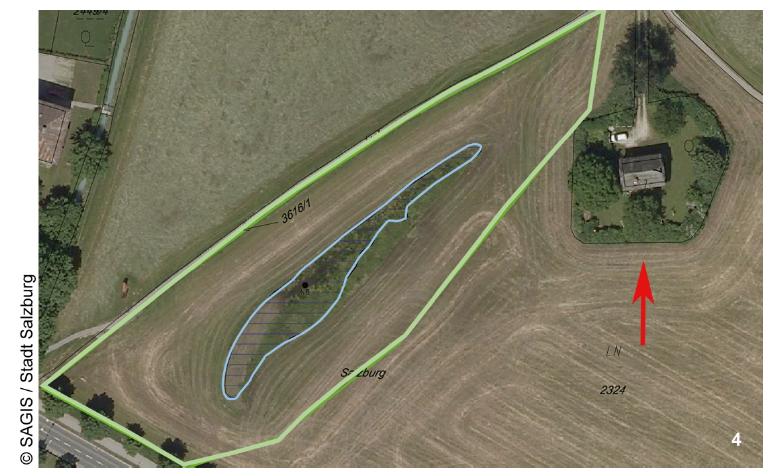
The newly protected feature is not that rocky bluff, Festungsberg hill, or the 11th-century fortress that sits on top of it. It is the long, narrow puddle in the foreground. This is Krauthügel Pond, an ephemeral body of water barely 30 cm deep where researchers have found 121 species of ciliates, ten of them previously undescribed. Because of these organisms–five of which have not been found elsewhere–Salzburg now possesses the world’s first second “Natural Monument for Single-celled Organisms.” A protist wildlife sanctuary!
The pond comes and goes during the year, appearing after heavy rains on a raised agricultural plot known as Krauthügel, or “cabbage hill.” The bed in which it lies is thought to be the remains of an old stream, whose natural course might have been altered by agriculture during the middle ages. Since then, roadwork and urban development have isolated the body from other surrounding channels.
From 1789 until 1960, the field was used for raising vegetables. After that, it became a pasture. For about thirty years, cows trampled the soft turf and nourished the local microbes with their manure, creating what ecologists call a “eutrophied pond.” In other words, a cattle wallow, or slough.
This is not what you could call a pristine natural environment. It doesn’t shelter any large, charismatic animals. It is not particularly scenic, when it can be seen at all (much of the year, the “pond” is dry). In short: it’s hard to imagine a patch of ground less likely to be singled out for conservation.
However, Krauthügel Pond has something your local ditches and mudholes lack: proximity to Wilhelm Foissner, an astonishingly productive ciliatologist who happens to live and work in Salzburg.
Arguably, the “natural wonder” here is not so much Krauthügel pond as Professor Foissner, whose vast body of work looms over modern ciliate systematics like the Festungsberg itself. With five or six hundred publications to his name–at least three hundred in peer-reviewed journals–Foissner, working alone or in collaboration, has discovered and described over 500 new protist species. If there were new ciliates in your cow field, he would be the man to find them.
Actually, to see a new species is not that unusual. Likely, we all run across undescribed organisms, from time to time, without knowing it. The little red bug that alights on your arm might be something never before recorded in the literature, if only you knew. Place samples of local mud under the microscope, and you are quite likely to find organisms that don’t yet have names. Of course, it’s one thing to see something new as it paddles by, and quite another to know what you have seen. To properly document your discovery, and publish the news of it, requires skills and technology that are in extremely short supply.
So, these ciliates were pretty lucky to have been born in Salzburg, near one of the few people in the world with the ability (and inclination) to see them for what they are and lobby for their protection. It raises some interesting questions.
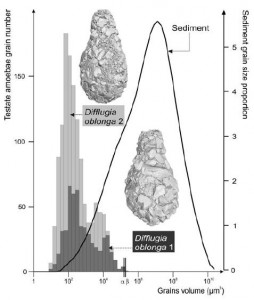
First, how exceptional is the microbe diversity that has been preserved in Krauthügel? In a report on the the pond, published earlier this year, Fenton P.D. Cotterill and his co-authors compare the ciliate species count at their location to various “well-investigated ephemeral waters” in other parts of the world: two meltwater ponds in Southwestern Ontario, a roadside puddle in Namibia, a rock pool in Venezuela, a meadow in Hungary, and several other choice spots. They find that the Salzburg pond is in the “upper range” for total number of species, but only in the “middle range” for the number of new species.
Evidently, the old cabbage field supports a high–but far from unique–diversity, and when closely probed by the best protistologists in the business, it yields about the expected number of new organisms. A rich but fairly ordinary body of water, it seems. Why single it out for protection?
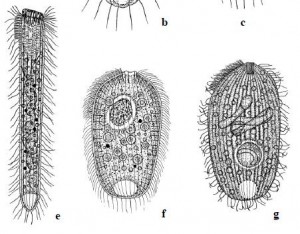
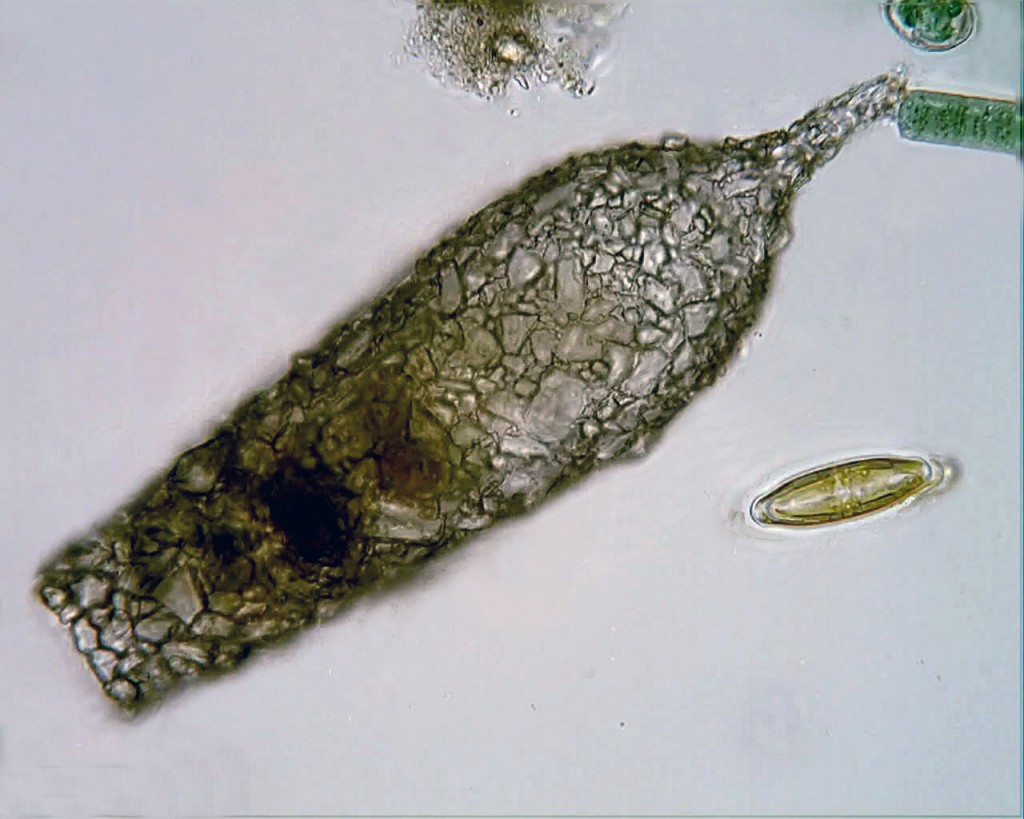
Three species only found at Krauthügel e) Semispathidium pulchrum f) Papillorhabdos multinucleatus g) Fuscheria nodosa salisburgensis
There are a couple of reasons. First, as the authors point out, appeals for conservation are usually based “on the narrow distribution of one or several species and their habitat, or of species and habitats endangered by human activities.” If a forest supports the only known population of Sibree’s Dwarf Lemur, we have reason to preserve it, because if we don’t, we can expect to lose that species forever. By that standard–provided we suppress the size-bias that can make us indifferent to the fate of a microbial species–the case for protecting Krauthügel is pretty strong. As of April, 2013, five of the the ten new species found there had “not been reported from any other locality.” Until they turn up elsewhere, those five species are assumed to be “endemic” to Salzburg (that is, restricted to that area). Given the scarcity of competent ciliatologists in other parts of the world, they may remain so for quite a while.
Whether they turn out to be truly endemic or not, it is indisputable that the organisms in the pond were “endangered by human activities”. In 2010, as part of an art project, somebody filled it in with earth. Imagine the alarm of researchers who had been studying the site for decades when they found out their protists had been buried! It was this event that prompted investigators to call for protection, resulting in the restoration of the pond to its previous condition and the creation of a buffer zone around it:
And that brings us to the second reason for conserving this puddle: thanks to the work that had already been done there, it has become the “type locality” for some eighteen species (eight new species, and ten redescribed taxa). The significance of this might require a bit of explanation.
When a biologist names a new taxon, the usual practise is to select a particular fixed specimen, or group of specimens, as the “type,” and (ideally) to deposit that specimen in a permanent collection somewhere, available to other researchers. This provides a permanent concrete reference, so there can be no ambiguity about what we really mean when we say Utricularia floridana (a species of carnivorous plant), or Amblyodus (a genus of beetle). If need be, we can point to a certain bug on skewered on a certain pin and say, “There! Amblyodus means that.”
The site at which the type specimen was collected becomes the “type locality,” where one might expect to find others of the same breed. That locality is especially important to protist taxonomists. Protists are small and fragile, and fixed type specimens of older named organisms are rarely available. Even when permanent slides exist, they can be lost, or simply deteriorate over time. If we know the type location where our guys were originally found, we can go look for them there. In theory. But if the place at which the work was done has been drained or paved, and no type material exists, the identities of the species found there can be lost in taxonomic noise.
What is being conserved at Krauthügel is, at least in part, the scholarly work that has already been done there. It is a body of acquired biological knowledge, and not just the organisms themselves, that is being protected. From this point of view, environmental conservation can be similar to task that museum and art conservators do, preserving the best products of human effort for future generations.
Where does that leave all the ponds that haven’t been, and likely never will be, studied? In spring, when I drive through the countryside where I live, I see ephemeral pools by the hundreds and thousands. They flash by in the car window, mile after mile: beaver ponds, ditches, mill pools, flood plains, and wide shallow puddles in fields where cows dip their muzzles and drop their nutritious poops. Some will have less protist diversity than Krauthügel, a few may have more, but none will ever enjoy the benign oversight of Wilhelm Foissner.
But what if more research were being done on these bodies of water–a protistologist for every puddle!–and more ponds found worthy of conservation? It is not clear where that road goes. If the Krauthügel initiative stirred up any controversy in Salzburg, there’s no record of it in the article, or the press release, but it’s not hard to anticipate the kind of pushback we’d see if similar initiatives were tried here. Attempts to control the use of private land arouse deep and incredibly long-lived resentments. Twenty-five years after efforts to conserve habitat for the Northern Spotted Owl in the PNW, anti-environmentalists are still seething and sneering. In some circles, the words “spotted owl” have become a kind of shorthand for “meddlesome tree-hugging morons who place a higher value on a stupid bird than the lives and livelihoods of hardworking humans.” Imagine the volcano of outrage that might erupt over the mandated protection of a one-celled organism! We would never hear the end of it.
All the same, the idea of protecting protist habitat has a lot of appeal for me. Down the road from my house in the Gatineau hills, there’s a group of ephemeral ponds where I like to gather samples. Within a few years, they will almost certainly be filled in, as the land is subdivided for new housing. I’ve watched those ponds for several seasons, and hate the idea of losing their amazing microscopic diversity. If, as is statistically probable, they contain a few new species, there might even be grounds for conservation. However, it is pretty certain that the bulldozers will get to any new organisms before I gain the competence and resources to find and describe them.
Still, I find it a little comforting to remind myself of the very different scale and speed of life at the microscopic level. When you are a hundred microns long, from tip to tail, a puddle is a lake, and a pond is an ocean. An hour is a year!
In a few days, a rain-filled tire rut can burst into startling diversity, like a miniature coral reef. Species bloom in quick succession, replacing and displacing one another. Each one changes the chemistry, light-permeability and nutrient load of the water, conditioning the environment to suit certain organisms, all of whom, in turn, will alter the water around them. Accidents of geography (a floating leaf, a ball of dung) make opportunities for some organisms, and extinguish all hope for others. Things progress quickly. If you return to the tire-rut every day and follow its progress with the help of a microscope, it can seem like looking at a time-lapse film. In the “big” world, environments change in much the same way, but over longer periods of time: forests encroach on prairies, then recede; wetlands take shape, silt up and vanish; animals come and go. At the microscopic level, shifts in populations may happen in hours, instead of years, and change is unremitting. “Ecological balance” is never achieved: things happen, then more things happen. Some species flourish, others fade from view. Then one day the hot sun dries it all up, and the little creatures climb back into their resting cysts, as their habitat reverts to grass.
———————————
References: