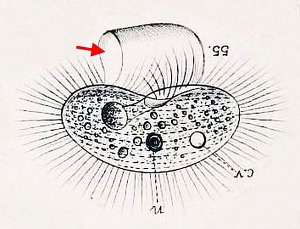
A few years ago, I looked in a sample of water from a bog lake, and saw something like a hyperactive avocado shifting around inside in a tiny kerosene lamp:
The architect of that pretty dwelling is the ciliate Calyptotricha pleuronemoides. The species and genus were discovered in 1882, in samples from a pond near Hertford, England, by an amateur naturalist named Frederick W. Phillips. Not much is known about him. During the 1880s, he was an active member of the Hertfordshire Natural History Society and Field Club, to whom he occasionally read essays on “The Protozoa of Hertfordshire,” based largely on the classification scheme in William Saville Kent’s Manual of the Infusoria. He was a Fellow of the Linnean Society of London, and he found and named a few new taxa.
In his very first glimpse of the creature, Phillips was lucky enough to catch it in the act of building its lorica. “At first sight,” he writes, “I thought it was an embryonic or encysted stage of some monad; but upon applying a magnifying power of some 900 diameters, I observed that it possessed a singular vibratile membrane, closely resembling that which characterizes the members of the family Pleuronemidae.” A week later, Phillips looked at it again, and discovered that “the lorica had increased in size, and that one end was elongated into a teat-like form.” At this stage, he accidentally allowed the sample to dry out, leaving the organism’s empty, half-finished lorica still attached to a strand of pond-weed. He made a nice drawing of what he’d seen.
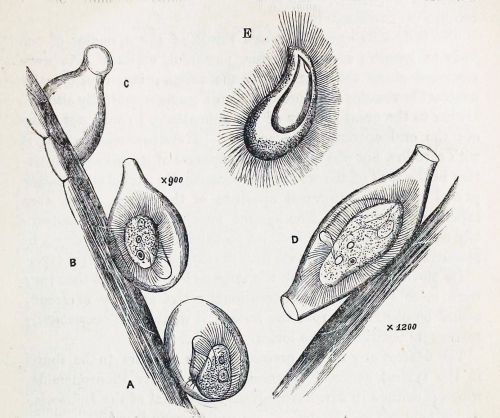
A. First stage B. The same, further developed C. End view of lorica D. The perfect animal E. Ventral view (adapted from Phillips)
To modern readers, accustomed to the impersonal, passive style of scientific writing–“samples were collected,” “living cells were isolated and observed”–there is something pleasingly candid about the way Victorian naturalists report their findings. Phillips doesn’t just describe his new genus, he spins us the tale of its discovery, including the mishap that destroyed his first specimen, and his initial misreading of the oval shell, after which he takes us to the very moment of discovery when he exposed the creature’s true nature by “applying a magnification of 900 diameters.” Something about that reminds me of the exploration literature of the same period. It’s probably not just an accident of style: Victorian microscopists were explorers. Superior lenses and stains had opened up a miniature Dark Continent on their laboratory benches, and a gentleman adventurer from somewhere like Hertfordshire could now penetrate these hidden realms, returning with breathless accounts of what he had seen. A session at the microscope was an expedition into the unknown.
In our time, researchers are expected to pile up some data before going to print, and nobody would attempt to erect a new ciliate genus on the basis of a brief observation of a few specimens. No doubt that is a good thing: the 19th century left a big legacy of poorly defined taxa, many of which are still desperately in need of revision. But this kind of field work, as sketchy and dilettantish as it might seem now, has largely been put to one side without really being replaced by anything better. Outside of a few centers of activity, ciliate field work has slowed to a crawl. Consider the fact that 132 years after Phillips wrote his three-page note on Calyptotricha pleuronemoides it is still one of only two substantial treatments of the species, and the only source that describes the construction of its curious lorica. Anyone who wants to know more about this ciliate than its name, has to travel back to the 19th century.
Needless to say, the old information is not always reliable.
Phillips perceived immediately, and rightly, that Calyptotricha is closely related to the more common ciliate Pleuronema. Like its cousin, it is equipped with a large, billowing membrane that runs along the right side of its oral aperture. However, Phillips badly misunderstood the shape of this structure, describing it as “a membranous trap, or velum, which in form resembled the old-fashioned poke-bonnet.”
When I first read that passage, the comparison to a “poke-bonnet” confused me. The undulating membrane of pleuronematid ciliates is shaped something like a sail, or a flag: a sheet of fused cilia running along one side of the organism’s mouth. Phillips, however, interpreted this structure (which, admittedly, is very difficult to see clearly in the light microscope) as a sort of hood or canopy covering the oral aperture of the ciliate. If you look closely at his illustration, you can see that he has drawn it as a baggy tube.
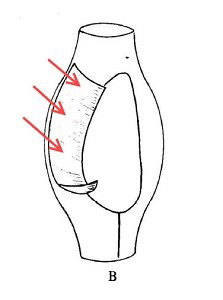
The true shape of Calyptotricha’s undulating membrane (image from Colin R. Curds, British and Other Freshwater Ciliated Protozoa, with arrows added)
Evidently, it was this imagined resemblance to a poke-bonnet that prompted him to give the genus its curious name, Calyptotricha, constructed from the Greek calyptos (“veiled” or “covered”) and trich (“hair”). It seems the “haired” holotrichous ciliate reminded him of a woman’s head, on top which the membrane sits like an old-fashioned hat!
It’s an example of how expectation shapes observation. In interpreting this membrane as an enclosed hood, he was deferring to an earlier error by his illustrious contemporary William Saville Kent. Writing about Pleuronema, Kent says: “[T]his membranous trap may be appropriately compared with the extensile hood of a carriage or an outside windowshade forming, when expanded, a capacious hood-shaped awning, and when not in use being packed away in neat folds close around the animalcule’s mouth.”
The “extensile hood” Kent mentions was a common convenience on carriages of his day, and provided a compelling mechanical analogy for the “neat folds” with which he imagined Pleuronema pulled back its velum.
 Here, for comparison, is Kent’s illustration of Pleuronema chrysalis, which I’ve inverted to showcase its “extensile hood.”
Here, for comparison, is Kent’s illustration of Pleuronema chrysalis, which I’ve inverted to showcase its “extensile hood.”
To modern workers familiar with the morphology of hymenostome ciliates, as revealed in specimens that have been stained with silver, this is an implausible design. However, to Kent, who had done pioneering work on choanoflagellates, it seemed reasonable to speculate that Pleuronema’s hood might share “a distant homological relationship” with the “delicate funnel-shaped membranes” found in the collared flagellates, which really do wear something a bit like a straw poke-bonnet (but on the back end of the cell).
Finally, since we’ve been talking about Pleuronema and her sisters, I’ll post some footage of one, quietly browsing on bacteria in water taken from a tidal pool on the coast of Maine: