There’s a natural pond down the road, where I collect samples. I always go to the same spot, the edge of a shallow basin in the “littoral zone”, where the pond shades into the shore. I follow the same routine each time: I dip a turkey baster into the soft muck–organic sediments enriched with duckshit and decomposing leaves–and suck out a few cc’s for the bottom of the jar; then I ladle a bit of surface water over the mud, to create a miniature approximation of the natural site.
I’ve taken hundreds of samples there over the years, from an area no bigger than a coffee table. And, despite having done this regularly for about half a decade, nearly every jar I bring home contains something I’ve never seen before.
Of course, to a microbe, a coffee table-sized site is actually quite big. If you were a typical one-celled protist–say, 50 micrometres from tip to tail–a four-foot patch of pond would be more than 24,000 times longer than your body. From a microbe’s point of view, then, my sampling basin is equivalent to a lake 28 miles long. Scaled up to human size, the entire pond would be at least 1,350 miles across, with a greater surface area than the Mediterranean Sea! So, there’s quite a lot of available habitat in the small body of water I call “Turtle Pond.”
At any given time, only a small fraction of the site’s potential diversity will be visible. The mix of species can change from one hour to the next, as weather and random events alter water chemistry, light levels, temperature and organic nutrients. A few hours of sunlight might kick off a sudden bloom of cyanobacteria, soon followed by nassulid ciliates that eat strands of blue algae like spaghetti; then, a few minutes of rain disperse the bloom, the nassulids disappear, and are replaced by some other combination of species. The new populations have barely established themselves, when a turtle (which, from a microbe’s point of view, is about 7 miles long), blunders into the shallows, churning up mud, mixing oxygen-rich upper layers with the anoxic sediments below, and everything changes again. Each shift in the pond creates new opportunities for some organisms, and makes life impossible for others. One species blooms, and another goes into dormancy, withdrawing into resting cysts or simply lurking in reduced numbers, waiting for better days.
The microbial “seed bank” can store a staggering diversity of such hidden opportunists, which emerge from their cysts only when conditions are exactly right. Some are “weed” species, generalists that crop up in nearly every sample. Others have very particular needs, and rarely appear. A few may never find what they need to grow and reproduce. If any cysts of salt-loving (halophilic) protists should blow in from some distant shore, they are probably out of luck.
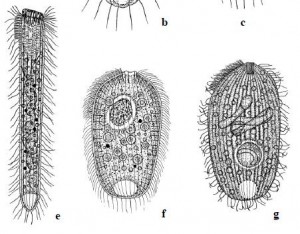
Recently, I brought home a few jars from the usual spot, and found three rather flamboyant species to cross of my life list. One was Actinobolina radians, an oddball I’ve been hoping to find for years (ever since I first saw it’s mugshot in Vladimir Schewiakoff’s beautifully-illustrated Infusoria Aspirotricha of 1889). Actinobolina is a ciliate in the class Litostomatea, a group of rapacious and morphologically hilarious carnivores. The class includes such celebrity ciliates as the improbably elastic Lacrymaria, the elephant-nosed Dileptus, and a frisky little keg full of death called Didinium, which eats other ciliates like popcorn. Like its cousins, Actinobolina is a hunter. However, it has a unique strategy for capturing prey. When swimming about, it looks much like any other ciliate: egg-shaped, and uniformly covered with cilia. Once it has a found a suitable feeding site, it comes to a stop and slowly extends a large number of long, stiff tentacles, each of which carries toxic extrusomes (cellular darts) to be released on contact into its victim’s body. In this state, it looks rather like a heliozoan “sun animalcule”. With its tentacles extended, Actinobolina lies in wait like a spider in its web, until some tasty creature–a rotifer , or another ciliate–bumps into it. The toxic extrusomes are deployed and the victim is paralyzed, whereupon the ciliate withdraws its tentacles, pulling the stunned prey toward its cellular mouth.
Unfortunately, I didn’t manage to record a successful hunt, but I did get some footage of the ciliate extending and retracting its tentacles. And really, I was just pleased to have seen this bizarre organism.
Another creature that appeared in my jars was the odd, oxygen-hating amoebozoan Pelomyxa, an indiscriminately hungry thing that looks like a tumbling bag full of rocks and food. I see Pelomyxa fairly often, but this one was a different species, smaller and more colorful, and with a distinctive style of movement, featuring frequent lateral “eruptions” of pseudopods. I believe it is Pelomyxa binucleata, a species first identified in the 19th century. For a time, in the late 20th century, it was believed that P. binucleata and all “species” of Pelomyxa, were really just forms and phases of a single species, Pelomyxa palustris. However, since 2004 the Russian researcher Alexander O. Frolov and his collaborators have been repopulating the genus, discovering new species and rehabilitating older ones. There are more than half a dozen, now.
Pelomyxa is also interesting because of its role in the rise and fall of the fabled Lost Protist Kingdom, once known as Archezoa. At one time, in the 1980s and early 90s, Pelomyxa was believed to be a primitive eukaryote, a “living relic of the Proterozoic era” (Margulis & Sagan, 1990) lacking such basic organelles as mitochondria, centrioles, flagella/cilia, endoplasmic reticulum, Golgi bodies and even chromosomes (Margulis & Chapman, Kingdoms and Domains, 2009). It was seen as sort of a missing link between eukaryotes and their bacterial predecessors, and was placed along with an assortment of other anaerobes, in the short-lived Kingdom Archezoa. However, that hypothesis began unraveling within just a few years. Pelomyxa were found to have centrioles and flagella after all, as well as mitochondrial remnants (“mitochondria-derived organelles”). In fact, they turned out to possess most of the stuff you’d expect to find in a eukaryotic cell, along with some nifty new adaptations to anaerobic living, so there was really no reason to regard them as “primitive” at all. When genetic analysis of the genus was finally done, it was placed squarely within the phylum Amoebozoa. Archezoa itself–briefly a great Kingdom, ruled by a benign despot named Thomas Cavalier-Smith–was quietly removed to the museum of obsolete evolutionary hypotheses.
Finally, my samples turned up a single specimen of Supraspathidium, a rather long haptorid ciliate with wide, blubbery lips. I’m afraid this guy will be of no interest to anyone but the most devout ciliatophile, but I’m posting it anyway because it’s my blog. Supraspathidium resembles ciliates of the better-known genus Spathidium, except that it has numerous contractile vacuoles, instead of a single big one in the posterior of the cell. This one has a long, wormy macronucleus, and generally resembles a spathidiid described by Eugène Penard in 1922,under the name Spathidium vermiforme.